Still Exploring?
Looks like you’ve been exploring our platform.
Want to see everything in one place?
The EU Medical Device Regulation (MDR) requires supply chain reporting to maintain market access. Assent supply chain sustainability platform helps complex manufacturers get there.

Assent has helped hundreds of companies. We’re proud to work with the following organizations.


























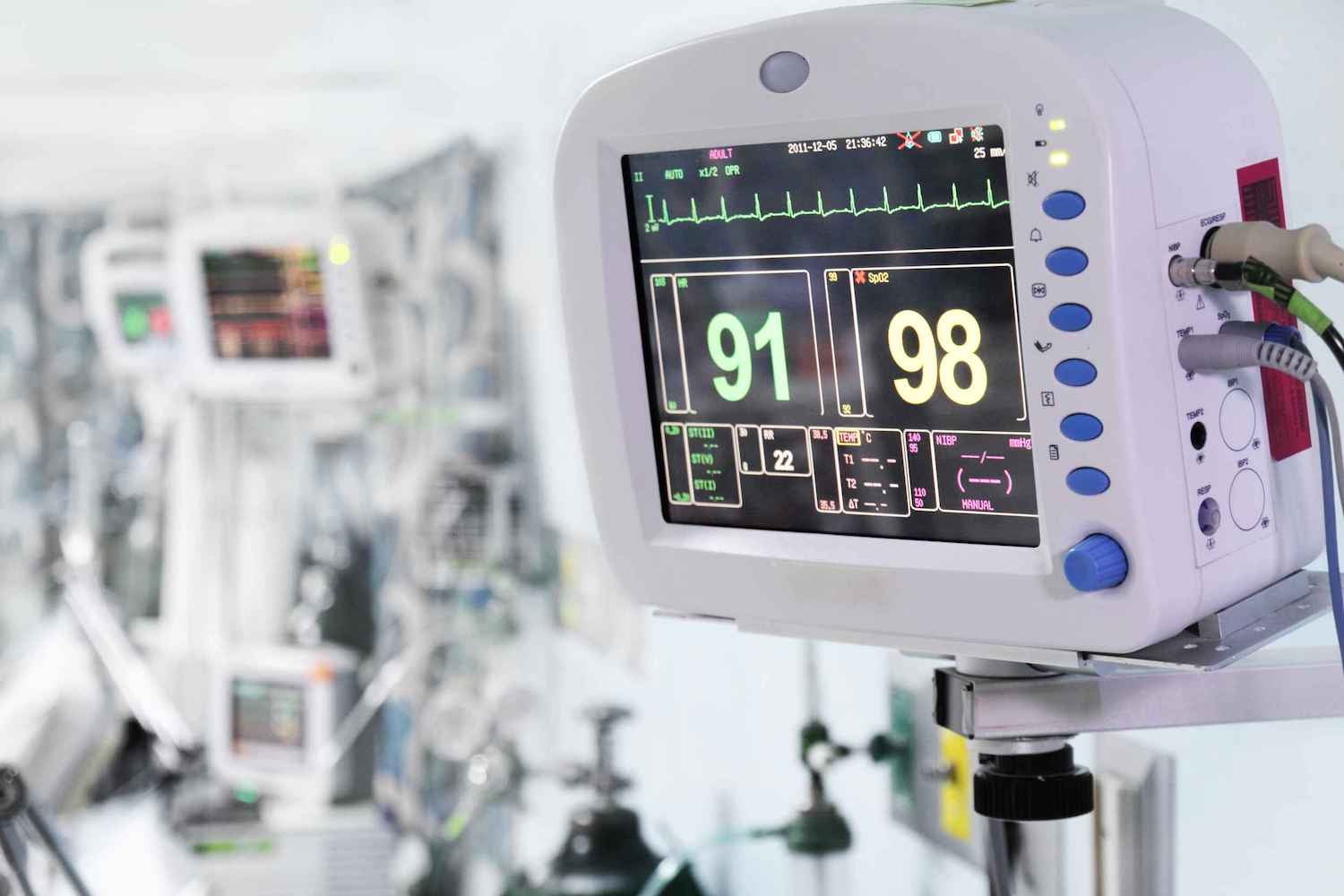
Medical device companies face unique and complex regulatory challenges — and the risks to your bottom line and reputation are high. When human health and safety are on the line, compliance can’t take a back seat.
This eBook explains the key regulations governing medical device manufacturing supply chains, along with insights on optimizing vendor management and corporate social responsibility.
Get the Ebook

Explore our solutions for getting standardized, validated, and usable product compliance data from your supply chain.