Still Exploring?
Looks like you’ve been exploring our platform.
Want to see everything in one place?
View our solutions for getting standardized, validated, and usable product compliance data from your supply chain.
Supply chain transparency needed for compliance.
Identify PFAS in your supply chain and set yourself up for success.
Meet submission obligations with a deep view of your supply chain.
Deep-map your supply chain to streamline compliance.
Grow better with Assent’s TSCA compliance solution.
Uncover deep supply chain data to meet labeling requirements.
Verify the origin of your metals to avoid costly fines and delays.
See how we build your missing ESG foundation with our complete supply chain sustainability solution.
Deliver on your reporting needs with Assent ESG
Uncover hidden forced labor risks in your supply chain.
Deep transparency into mineral sourcing.
Easily see and manage the embedded emissions data of your goods.
Meet your German Supply Chain Due Diligence Act (SCDDA) requirements with confidence.
Confidently identify the entities and topics in your value chain that are most critical to compliance.
Learn about our trade compliance capabilities and how it uncovers hidden risks and opportunities.
Unlock supply chain transparency to mitigate trade risks.
Manage customs codes for your products.
Protect global market access & leverage trade agreements.
Verify the origin of your metals to avoid costly fines and delays.
Solutions for export classification code tracking.
Country of origin tracking supply chain solutions.
Discover how our automated supply chain sustainability management solutions help complex manufacturers see deeper, be smarter, and grow better.
Solutions for medical devices manufacturing.
Solutions for the electronics industry, across compliance and ESG.
Sustainability solutions for industrial equipment.
Supply chain sustainability for automotive.
Sustainability solutions for aerospace and defense.
View details about our complete supply chain sustainability platform, including product compliance, ESG, and trade compliance.
Evaluate and report on hidden risks in your supply chain.
Get a custom quote and find the right solution at the right price.
Learn about our data security protocols, to keep your information safe.
Reporting and data capabilities you need to meet your requirements.
View our integrations with SAP, PTC Windchill, and other standard APIs.
View details about our complete supply chain sustainability platform, including product compliance, ESG, and trade compliance.
Education, data collection, and technical support for suppliers.
Deep regulatory knowledge you need to build sustainable growth.
Managed services with dedicated support from world-class experts.
Assent regulatory experts have over 200 years of combined expertise. Learn more about how they helped make us the leader in supply chain sustainability.
View our content hub, including Interactive Tools, Webinars, Whitepapers, Guides and eBooks.
New Tools for understanding your requirements, risks, and our solutions.
Explore complex supply chain sustainability topics.
Get up-to-date information on constantly evolving topics.
Keep on top of existing or emerging regulations and trends.
Learn directly from experts on wide-ranging topics.
Discover why so many top companies have chosen Assent.
Access the Assent Knowledge Center, the leading regulatory resource for supply chain management, from our team of experts.
New Per- and polyfluoroalkyl substances (PFAS)
New Streamline your reporting process to comply with the EU CSRD.
Deep transparency into mineral sourcing.
Supply chain transparency needed for compliance.
Environmental, social, and governance (ESG) issues.
Deep-map your supply chain to streamline compliance.
The German Supply Chain Due Diligence Act (SCDDA) .
Grow better with our TSCA compliance solution.
Read Assent’s blog for regulatory updates, supply chain sustainability advice, best practices, and more.
Learn everything you need to know about supply chain sustainability.
Get clear educational resources on key product compliance regulations.
Discover how ESG and your supply chain are connected.
Learn best practices for managing your trade requirements.
Keep up with changing responsible minerals expectations.
Get an expert breakdown of the REACH Regulation.
Read about upcoming events and webinars hosted by our world-class team of regulatory experts.
Access comprehensive educational resources to better understand your business and regulatory requirements, and how to build your programs to mitigate supply chain risk effectively.
Learn how we became the leading supply chain sustainability management solution for the world’s most responsible complex manufacturers.
Read about our ESG journey and access our corporate sustainability reports.
See the leading manufacturers working with us to achieve supply chain sustainability.
Meet the team that helped Assent become the leader in supply chain sustainability.
Read the latest news about Assent from around the world.
View our industry and technology certifications.
Read more about the organizations and partners working with us to create supply chain sustainability.
Find out how to get in touch with us.
Become an Assentee! Learn about our culture, purpose, and opportunities.
A Brexit FAQ for Companies Based in the UK & European Union
In 2017, the UK government triggered Article 50 of the Treaty of Lisbon initiating the British exit (Brexit) procedure. On January 31, 2020, the UK will officially leave the European Union (EU) following years of negotiations and several extensions. This will have wide-ranging regulatory impacts on businesses in both the UK and EU, and change the international trade landscape.


Brexit will bring fundamental changes to manufacturers, importers and distributors in the UK and EU as new regulations are established and requirements change. We have answered some of the most frequently asked questions that companies are asking about Brexit.
The UK and the EU have agreed on a transition period that will maintain the status quo for businesses until the end of the 2020 calendar year. The UK government has stated its plan to reach a trade agreement with the EU by the end of 2020. This agreement is expected to provide more information on the regulatory and trade landscape after 2020. While much is still unknown, companies are advised to use the transitional period to adopt processes enabling compliance, such as obtaining substance-specific part-level data from their suppliers.
Brexit is a large-scale change for the UK regulatory landscape and will lead to many new processes for businesses, which may not yet be fully defined. The most significant regulatory changes emerging from Brexit are with respect to substance regulations such as the Restriction, Evaluation, Authorisation and Restriction of Chemicals (REACH) Regulation. As an EU regulation, REACH will no longer apply to the UK once it leaves the EU.
This is different from a directive, which is a set of guidelines individual member states are required to transpose into national law. For example, the UK government transposed the Restriction of Hazardous Substances (RoHS) Directive into national law, and it will remain in effect post-Brexit, but with a different legal base.
Companies in the UK will no longer be in scope of EU REACH. However, they must abide by the rules laid out in the regulation to maintain access to the EU market.
If a substance was registered for an intended use by a UK company, that registration will no longer be valid. UK companies wishing to conduct business in the European Economic Area (EEA) must register the substance through an Only Representative (based in the EU), or relocate the business operations that are responsible for the substance to an EU member state and register there. Joint registrations with a UK-based company as the lead registrant will also be invalid post-Brexit.
The relevant staff and documentation must be present at the address listed on a substance registration dossier. A registration will be invalid if the listed address has no operational relevance to the substance.
Yes, but they must comply with EU regulations such as REACH. To place products on the EEA market, companies must demonstrate compliance. It is currently unclear how UK companies can accomplish this. Unless the UK and EU reach an agreement with unique stipulations, UK companies must follow the same process as other non-EU companies in order to access the EEA. This involves establishing a relevant office in an EU member country or using an EU-based Only Representative.
After Brexit, UK and EU regulatory bodies will operate independently, unless a formal alignment is achieved in a trade deal. The UK REACH system is the proposed replacement to the European Chemicals Agency (ECHA) REACH-IT system used for EU REACH. Developed by the UK Health and Safety Executive, and the Department for Environment, Food and Rural Affairs, UK REACH can be used to:
The UK REACH-IT system is currently in its early stages and is on hold awaiting the outcome of trade negotiations.
Companies have 120 days from exit day (January 31, 2020) to register a substance
The regulatory requirements established by EU regulations will not change for EU-based companies as it pertains to their business within the EU market. European Union companies wishing to enter the UK market must align with the laws of that country. To comply with UK REACH rules, EU companies can leverage a UK-based Only Representative to register substances.
If an EU company is supplied substances from a UK-based entity, the substances imported into the EU must be registered by an Only Representative or the EU importer. Substances registered by UK-based companies are invalid as of the Brexit date.
The CE markings used to demonstrate compliance will still be used on products in the UK during the transition period. If no deal is reached before the end of this period, the UK has developed the UK Conformity Assessed (UKCA) marking for certain goods. The UKCA marking will apply for products that:
The EU will not recognize the UKCA marking and CE markings will still be required to enter the European market.
The current expectation is that the UK will leave the EU customs union, so trade will be subject to any relevant tariffs and duties. As mentioned, the UK government has expressed an aim to finalize a free trade agreement with the EU before the end of the year.
Trade deals already struck between countries and the EU will only affect EU member state countries, not the UK. However, the UK can now negotiate free trade deals with non-EU countries independently. Any trade deals established this way will not take effect until January 1, 2021.
Additionally, safety and security certificates will be required for goods leaving the UK for the EU. Entry and exit forms will be required for goods traveling from Northern Ireland to Great Britain, even though this is a domestic trade route
Brexit has no direct impact on the U.S. However, as the UK and the EU are now separate blocs, U.S. companies face more complexity in demonstrating compliance. For example, after the transitional period, in a no-deal scenario, a U.S. company may have to provide two registrations for a substance’s specific use to both EU REACH and UK REACH entities. Companies around the world must also maintain accurate and detailed records of country of origin for their parts, as trade deals with the EU will no longer apply to parts originating from the UK.
The Assent Compliance Platform enables a proactive approach to regulatory changes such as Brexit. Learn how your company can leverage automation and centralization to prepare for a range of post-Brexit data requirements.
Download Guide

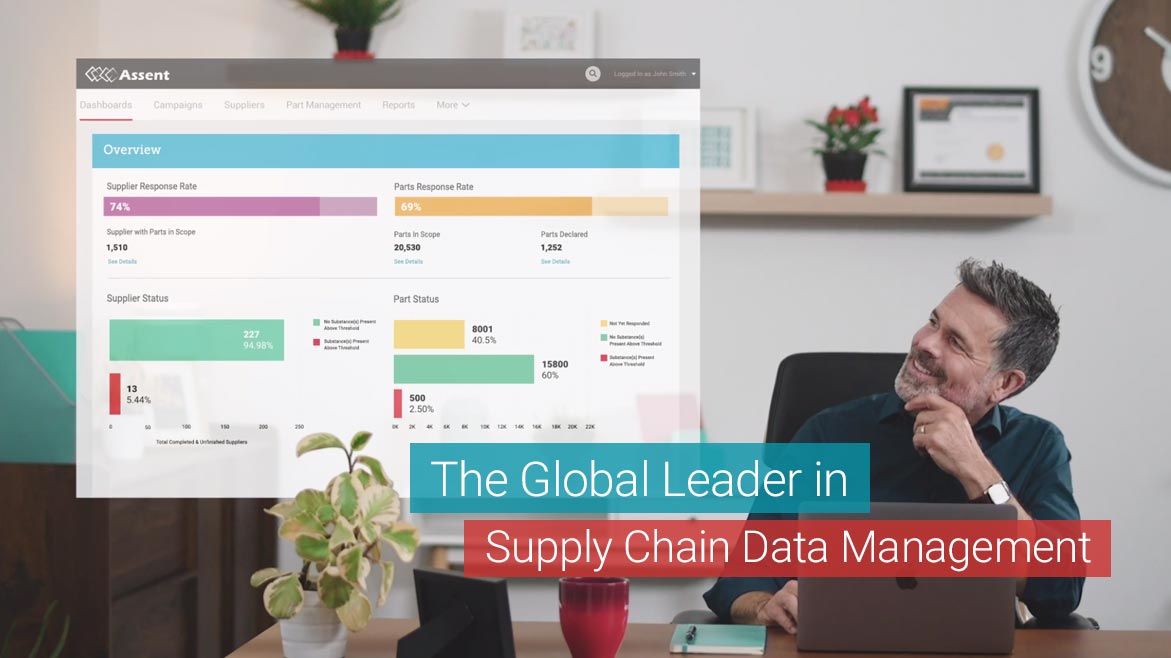

The post-Brexit transition period gives companies time to develop processes for new data requirements emerging from the UK’s departure from the EU. Assent Compliance empowers companies with the industry-leading supply chain data management platform, so they can be prepared to maintain market access after the Brexit transition period ends.
Leveraging this data, companies can efficiently demonstrate compliance with both UK and EU substance regulations, track country of origin information and identify risks in the supply chain. Assent also has a world-renowned team of regulatory experts that offer guidance through the complex post-Brexit process.


Looks like you’ve been exploring our platform.
Want to see everything in one place?