Still Exploring?
Looks like you’ve been exploring our platform.
Want to see everything in one place?
The EU Medical Device Regulation (MDR) requires supply chain reporting to maintain market access. Assent supply chain sustainability platform helps complex manufacturers get there.
Assent has helped hundreds of companies. We’re proud to work with the following organizations.








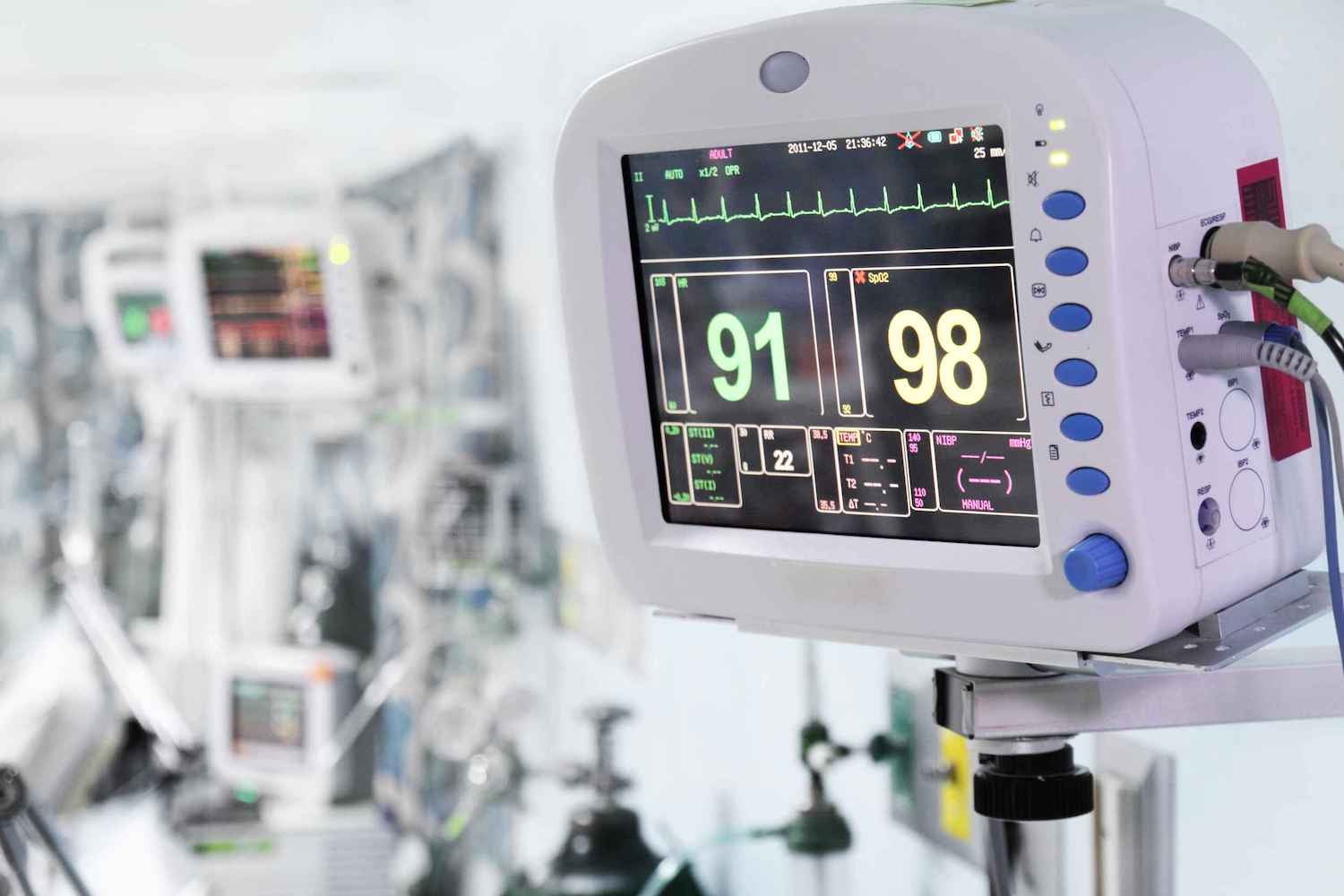
Medical device companies face unique and complex regulatory challenges — and the risks to your bottom line and reputation are high. When human health and safety are on the line, compliance can’t take a back seat.
This eBook explains the key regulations governing medical device manufacturing supply chains, along with insights on optimizing vendor management and corporate social responsibility.
Get the Ebook
Assent’s EU MDR solution allows manufacturers to deep-map the complex manufacturing ecosystem, unlocking the ability to survey suppliers at the part level for substances of very high concern (SVHC) data, gain deep visibility into supply chain risk, and support IPC-1752A Class D XML imports.
Protect your market access and ensure you meet your EU MDR requirements by seeing deep into your supply chain to examine suppliers, their products, and even substances within parts.
To maintain brand reputation and protect against recalls, you need to ensure your products are trustworthy and safe. Assent will collect the hard data you need to get there, going down to the root of what’s material in your products.
Reassign internal resources from data collection to revenue-boosting activities, ensuring you meet your business goals.
Explore our solutions for getting standardized, validated, and usable product compliance data from your supply chain.